RNA-based therapies are enjoying a renaissance, owing in part to the recent approvals of Alnylam Pharmaceuticals’ RNA interference (RNAi) drugs, Onpattro® (patisiran) and Givlaari™ (givosiran). RNA-based drugs are also gaining traction because their use to silence gene translation does not involve editing DNA. Such drugs have been approved before for use in the clinic, particularly for the treatment of patients infected with human immunodeficiency virus (HIV). Although many different approaches exist for making use of RNA for therapy, this tutorial will focus on short hairpin RNA (shRNA), an artificial RNA molecule with a tight hairpin turn, and its potential use in developing chimeric antigen receptor (CAR) T-cell therapies.
Why CAR T cells?
CAR T-cell therapy is able to program a patient’s immune system to target cancer, by genetically modifying T cells to express a receptor, designed to recognize and bind to specific tumor antigens, in a personalized approach that offers the potential to overhaul the current treatment paradigm of nontargeted chemotherapy. Following tumor antigen binding, the T cell can destroy the targeted cancer cells.
There are currently over 800 CAR T-cell clinical trials in progress, with some therapies showing up to 94% remission rates for the most severe forms of blood cancer.1 Although CAR T-cell therapies show promise, they have yet to be widely adopted in the clinic. Adoption has been slow because these therapies face significant challenges such as lengthy manufacturing processes and potentially lethal side effects.
There are two distinct types of CAR T-cell therapy: autologous and allogeneic. Using a patient’s own T cells, autologous therapies reduce the risk of rejection by the body. However, the patient-specific nature of autologous therapies means manufacturing CAR T cells is a time- and labor-intensive process. Autologous therapies are expensive and can take weeks to manufacture.
Allogeneic therapies, or "off the shelf" therapies, can overcome these limitations, as CAR T cells can be manufactured at scale from an external source and stored until needed by patients. However, a major drawback is that the implanted cells are recognized as foreign by the patient’s immune system. This means the body may reject and attack them, resulting in a loss of therapeutic impact. Worse still, the CAR T cells can also reject the host, attacking the patient’s noncancerous cells in a potentially deadly complication known as graft-versus-host disease (GVHD).
Controlling GVHD in allogeneic CAR T-cell therapies is key to making safe, off-the-shelf therapies readily available. One approach is to target the activity of the T-cell receptor (TCR) molecules expressed on the surface of CAR T cells that are largely responsible for the rejection of host tissues in GVHD.2
Gene editing versus genetic modulation
Researchers have employed gene editing techniques such as zinc finger nucleases (ZFNs), transcription activator like effector nucleases (TALENs), or CRISPR nucleases to knock out unwanted genes.3 These have associated risks, however, owing to the insertion of DNA double-strand breaks within the genome.
Tailoring gene expression in CAR T cells using shRNA is also being explored. Because shRNA-based gene silencing is long-lasting, and because other kinds of shRNA therapeutics have been clinically approved, shRNA-based gene silencing treatments allow for relatively straightforward Investigational New Drug (IND) applications and regulatory approvals. That is, the regulatory demands accompanying gene silencing based on shRNA are less daunting than those accompanying gene silencing based on gene editing. Although shRNAs can also exhibit off-target effects, developments over the years have identified ways of limiting these. For example, an increasingly common strategy is the incorporation of microRNA scaffolds.4
This strategy is being executed by Horizon Discovery through its SMARTvector™ bioinformatics platform. SMARTvector can facilitate the analysis of data from large-scale knockdown experiments and bespoke algorithms, the design of potent shRNA sequences, and the insertion of shRNA sequences into a microRNA scaffold (an optimized version of miR-196-a2).
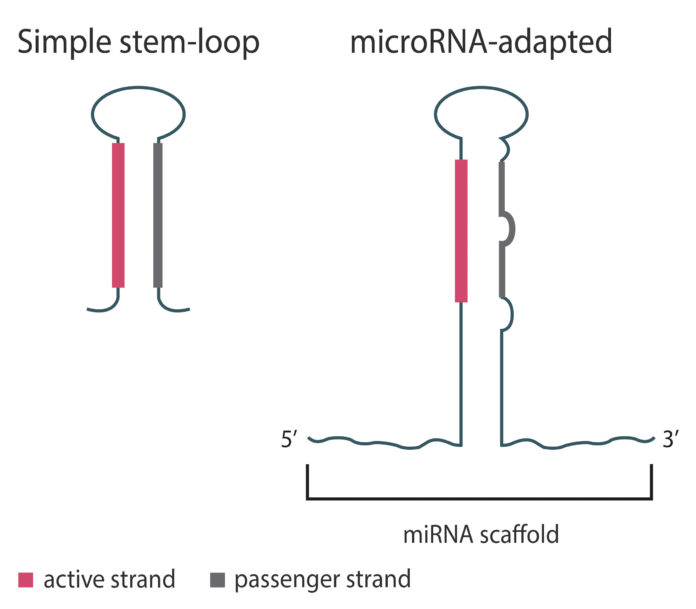
The platform produces “microRNA-adapted shRNAs” that can advance the development of less toxic and more tunable shRNA modalities by favoring function of the active strand, which targets genes of interest. Without an accompanying microRNA scaffold (simple stem-loop shRNA, (Figure 1), the passenger and active strands are equally potent. This can result in greater toxicity due to off-target effects from the passenger strand, and the need for higher doses to achieve a therapeutic effect.
shRNAs and CARs
As the CAR T-cell field has progressed, approaches for disabling TCR activity have largely used gene editing techniques. Though this is an attractive strategy, these methods do not achieve 100% knockout, and there is potential for dangerous off-target effects.3 Moreover, therapeutic applications involving the gene editing techniques are heavily regulated,5 more heavily than therapeutic applications involving shRNA-based gene silencing.
In October 2018, Celyad initiated a collaboration with Horizon to employ the SMARTvector shRNA technology as a T-cell engineering strategy to limit GVHD in patients treated with allogeneic CAR T cells. Gene silencing using shRNA has now become Celyad’s primary T-cell engineering strategy.
Celyad’s new CAR T-cell therapies are in preclinical development. They use shRNA sequences to target the endogenous gene encoding full-length CD3ζ, a co-stimulatory molecule that is essential for TCR function. Studies are ongoing regarding the efficacy of this approach, but preclinical studies, which have yet to be peer reviewed and published, show promise. 4
In CYAD-101, the company’s first allogeneic CAR T-cell therapy to be investigated for solid tumors, TCRs are inactivated using inhibitory peptides. This approach is being tested in a Phase I trial for relapsed/refractory metastatic colorectal cancer, concurrent with FOLOX chemotherapy. No cases of GVHD have thus far been reported.2
Celyad’s CYAD-02, an shRNA-modified CAR T-cell therapy, utilizes SMARTvector shRNA technology. CYAD-02 has recently been approved by the FDA as an IND, with a Phase I trial scheduled for early 2020. The trial will assess the safety and efficacy of CYAD-02 in a dose-escalation study, involving a preconditioning chemotherapy in patients with relapsed/refractory acute myeloid leukemia or myelodysplastic syndromes.
Future perspectives
Some of the key issues that limit the use of allogeneic CAR T cells, such as GVHD, can be addressed using gene modulation technologies such as shRNA silencing. The outlook for this approach is promising. Associated off-target effects are well characterized and can be limited by employing microRNA. Thus far, they have not been a barrier to regulatory approval. The full nature of off-target effects associated with gene knockout is still being established, making the regulatory route for approval more complex. Thus, shRNA could enable safer and more effective CAR T-cell therapies to enter the therapeutic pipeline, and potentially the clinic, more quickly.
Ensuring that CAR T cells persist in the body long enough to achieve a therapeutic effect, and preventing resistance to CAR T-cell therapies are just some of the issues that face the field. Manufacturing autologous CAR T cells remains a challenge as some patients’ T cells are simply not fit for purpose. The use of an off-the-shelf allogeneic CAR T-cell therapeutic could help, but the issues of GVHD cannot be ignored.
An allogeneic solution might be possible using older technologies that circumvent the concerns around off-target effects introduced by gene editing. However, the race is still on in terms of finding cellular solutions that can deliver therapeutic efficacy in an off-the-shelf format. We are only in the first iterations of these promising advances, and clinical trial data alone will determine successful routes to the clinic for the rapidly evolving approaches being used to improve CAR T cells.
Nicola McCarthy, PhD, is screening business unit manager at Horizon Discovery.
References
1. Fernández CR. A Cure for Cancer? How CAR T-Cell Therapy is Revolutionizing Oncology. Published January 2018. Accessed March 9, 2020.
2. Letter to Shareholders—January 2019. Published January 07, 2019. Accessed March 9, 2020.
3. Graham C, Jozwik A, Pepper A, Benjamin R. Allogeneic CAR-T Cells: More Than Ease of Access? Cells 2018; 7(10): 155.
4. Tuzman, KTT. Second shot for shRNA: How Tool Developers and CAR T Cell Companies Could Recharge shRNA Therapies. Published April 4, 2019. Accessed March 9, 2020.
5. Evitt NH, Mascharak S, Altman RB. Human Germline CRISPR-Cas Modification: Toward a Regulatory Framework. Am. J. Bioeth. 2015; 15(12): 25–29.
Ubigene Biosciences is co-founded by biological academics and elites from China, the United States, and France. We are located in Guangzhou Science City, which serves as a global center for high technology and innovation. Ubigene Biosciences has 1000㎡ office areas and laboratories, involving genome editing, cell biology technology, and zebrafish research. We provide products and services for plasmids, viruses, cells, and zebrafish. We aim to provide customers with better gene-editing tools for cell or animal research.
We developed CRISPR-U™ and CRISPR-B™(based on CRISPR/Cas9 technology) which is more efficient than general CRISPR/Cas9 in double-strand breaking, CRISPR-U™ and CRISPR-B™ can greatly improve the efficiency of homologous recombination, easily achieve knockout (KO), point mutation (PM) and knockin (KI) in vitro and in vivo.
Genome Editing Platform
——Focusing on the Application of CRISPR-U™ and CRISPR-B™ Gene Editing Technology
Cell Biology Platform
——Focusing on primary cell
2. Provides culture strategies and related products for different cell types.3. Provides cell biology-related services such as cell isolation, extraction and validation.

